This is online E log book to discuss our patient's de-identified health data shared after taking his/her/guardian's signed informed consent. Here we discuss our individual patient's problems through series of inputs from available global online community of experts with an aim to solve those patients clinical problems with collective current best evidence based inputs.This e-log book also reflects my patient centered online learning portfolio and your valuable inputs on comment box is welcome.
E.Laharika
Roll no: 29
- I've been given this case to solve in an attempt to understand the topic of "patient clinical data analysis" to develop my competency in reading and comprehending clinical data including history, clinical findings, investigations and come up with a diagnosis and treatment plan.
CASE PRESENTATION
32 year old male presented to casualty 1 week back with chief complaints of:
- 1 episode of seizures ( involuntary movements of Bilateral upper and lower limbs)
- Involuntary micturition
- Burning micturition
HISTORY OF PRESENTING ILLNESS:Patient was apparently asymptomatic 1 week back,then he developed
1 episode of seizures (GTCS) which lasted for 10 min associated with nystagmus,frothing at the mouth and tongue bite.
He had post ictal confusion for 10 minutes.
No h/o head ache/ vomiting/ fever
Patient also has hyperpigmented scaly patches all over the body associated with itching since 1 year.
PAST HISTORY:
Patient is a known case of Diabetes mellitus since 2 years.
No history of HTN
No history of any previous epilepsy
In November 2020:
He visited the hospital with complaints of Bilateral loin pain and was diagnosed with Bilateral ureteric calculus with right renal calculus.
2 sessions of hemodialysis were done in view of post renal AKI and discharged on medical treatment.
PERSONAL HISTORY:
Diet - Mixed
Appetite- Normal
Sleep- Adequate
Bowel and bladder movements- involuntary micturition
No Addictions
FAMILY HISTORY: Not significant
GENERAL EXAMINATION:
The patient is coherent cooperative and conscious, well oriented to time place and person
He is moderately built and Nourished.
Pallor +
No icterus
No Cyanosis
No clubbing
No lymphadenopathy
No Edema
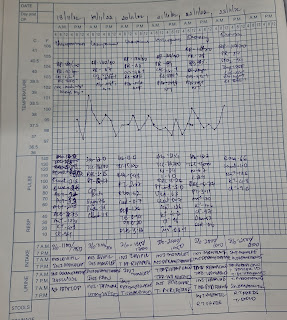
Vitals on 16 /9 /21:
Temperature-Afebrile
BP- 110/80 mm hg
PR -99 bpm
Sp02 - 99% on RA
GRBS- 191 mg/dl.
SYSTEMIC EXAMINATION:
CVS - S1 S2 Sounds are heard
RS - BAE +
P/A - non tender
CNS- speech is normal
Reflexes are normal
Motor and sensory system-normal
Cranial nerves-intact
INVESTIGATIONS on 10 /9/21:
HEMOGRAM :
ABG :
pH - 7.04
pCO2 - 13.9
pO2- 107
HCO3- 6
Patient was taken on hemodialysis in the view of severe metabolic acidosis.
CUE :
Albumin - ++++
ALKALINE PHOSPHATASE :
SERUM PROTEIN :
SERUM ALBUMIN :
SERUM ELECTROLYTES :
TOTAL PROTEINS A/G RATIO :
ANTI HCV ANTIBODIES - RAPID:
HBsAg- RAPID
HIV 1/2 RAPID TEST:
SERUM CREATININE:
BLOOD UREA:
SERUM BILIRUBIN:
SGPT:
SGOT:
Patient is referred to dermatology for examination of hyperpigmented patches all over the body.
Impression: Found to be infected with TINEA CORPORIS et CRURIS et FACIEI.
ULTRASOUND REPORT:
Impression:
Mobile coarse debris noted in the urinary bladder.
Bilateral hydroureteroneohrosis ( moderate to severe)
Bilateral DJ stents insitu.
PROVISIONAL DIAGNOSIS:
Uremic Encephalopathy
CKD on MHD associated with Bilateral DJ stenting insitu
TINEA CORPOTIS et CRURIS et FACIEI.
TREATMENT:
DAY 1
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. INJ.CEFTRIAXONE 1 gm IV/BD
4. Tab. LASIX 40 mg BD
5. Tab.NODOSIS 500 mg TID
6. Tab. SHELCAL 500mg OD
7. Tab. OROFER BD
8. GRBS monitoring 6th hourly
9. Inj. NaHco3 150mg 100ml NS IV/over 1 hr
10. Strict I/O charting
11. BP/PR/Temperature/Spo2 monitoring
12. Inj.HAI s/c according to GRBS
14. Inj. LEVIPIL 500mg IV/BD
DAY 2
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj.LEVIPIL 500 mg IV/ BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7.GRBS monitoring 6th hourly
8. Tab. NODOSIS 500 mg BD
9. Tab. Bio d3 500mg BD
10. Tab. LIVOGEN BD
11. Strict I/O charting
12. BP/PR/Temperature/Spo2 monitoring
DAY 3
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj. LEVIPIL 500mg IV/BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7. GRBS monitoring 6th hourly
8. ZODERM -E lotion L/A BD
9. Tab. NODOSIS 500 mg BD
10. Tab. Bio d3 500mg BD
11. Tab. LIVOGEN BD
12. Strict I/O charting
13. BP/PR/Temperature/Spo2 monitoring
DAY 4
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj. LEVIPIL 500mg IV/BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7. GRBS monitoring 6th hourly
8. ZODERM -E lotion L/A BD
9. Tab. NODOSIS 500 mg BD
10. Tab. Bio d3 500mg BD
11. Tab. LIVOGEN BD
12. Strict I/O charting
13. BP/PR/Temperature/Spo2 monitoring
DAY 5
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj. LEVIPIL 500mg IV/BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7. GRBS monitoring 6th hourly
8. ZODERM -E lotion L/A BD
9. Tab. NODOSIS 500 mg BD
10. Tab. Bio d3 500mg BD
11. Tab. LIVOGEN BD
12. Strict I/O charting
13. BP/PR/Temperature/Spo2 monitoring
DAY 6
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj. LEVIPIL 500mg IV/BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7. GRBS monitoring 6th hourly
8. ZODERM -E lotion L/A BD
9. Tab. NODOSIS 500 mg BD
10. Tab. Bio d3 500mg BD
11. Tab. LIVOGEN BD
12. Strict I/O charting
13. BP/PR/Temperature/Spo2 monitoring
DAY 7
1. Fluid restriction <1.5 ltrs/day
2. Salt restriction <2 gms/day
3. Inj. LEVIPIL 500mg IV/BD
4. Inj. CEFTRIAXONE 1 mg IV/BD
5. Tab.Lasix 40 mg BD
6. Inj.HAI s/c according to GRBS
7. GRBS monitoring 6th hourly
8. ZODERM -E lotion L/A BD
9. Tab. NODOSIS 500 mg BD
10. Tab. Bio d3 500mg BD
11. Tab. LIVOGEN BD
12. Strict I/O charting
13. BP/PR/Temperature/Spo2 monitoring
























Comments
Post a Comment